Basic HTML Version




August 2013 |
drinkanddrugsnews
| 15
www.drinkanddrugsnews.com
Tobacco |
Harm reduction
Where to with tobacco policy?
Harm reduction is central to current tobacco policy in England. This will be
welcome news to those in the drugs field who feel rather beleaguered and
browbeaten by the drugs recovery agenda.
The history of tobacco harm reduction is tied in with the development of NRT. In
1990 there were only three NRT products, all prescription-only. Pharmacy sales
started in 1991, expanded to all products by 2000, and since 2009 all NRT products
have been on general sale. Initially NRT was only indicated for abrupt quitting, and
later extended for people who wanted to cut down more gradually before stopping.
But in 2009/2010 the MHRA agreed it could be used for harm reduction, including
temporary abstinence and a reduction in smoking with no intention to quit.
The Department of Health’s 2011 tobacco control plan committed to
encouraging tobacco users who cannot quit to switch to safer sources of nicotine
and to encourage manufacturers of safer sources of nicotine to develop new types
of nicotine products. In 2012 the Cabinet Office’s behavioural insights team – the
so-called ‘nudge unit’ – urged the use of e-cigarettes.
The most recent piece of the story is the work of the National Institute for Health
and Clinical Excellence (NICE) which gives a strong endorsement for tobacco harm
reduction for people who do not wish to quit smoking altogether, and for people
who want to quit smoking but are unable or unwilling to quit using nicotine. The
NICE group concluded that nicotine does not pose a significant health risk.
Enter MHRA and the European Tobacco Products Directive. The MHRA has
decided to regulate all electronic cigarettes as medicinal products by 2016, and
the European Tobacco Products Directive – currently going through the European
legislative process – proposes that all e-cigarettes should be regulated under
medicines legislation.
‘Smoking cessation’ or ‘smoking sensation’?
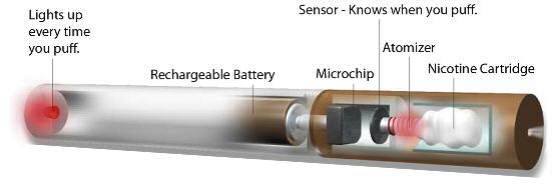
Those in favour of medical regulation argue that electronic cigarettes are currently
unregulated products, that they are accessible to children, that there is no control
over advertising, that they contain potentially dangerous constituents, and that the
devices themselves, including the batteries, pose a threat to user safety.
Medicines regulation, they argue, will improve safety, quality and efficacy, and
make them work better as a smoking cessation product.
There is another thread going through the political argument however, which
is that electronic cigarettes are just another way to feed addiction to nicotine, and
that they send the wrong message and undermine attempts to drive down
tobacco use. Some claim that electronic cigarettes are contrary to efforts to ‘de-
normalise’ smoking. There are already scaremongering stories about
schoolchildren using them. Certainly they might be a short-term fad amongst
some children who wish to challenge authority, but the e-cigarettes market is
made up of long-term smokers and surveys by Action on Smoking and Health
(ASH) show minimal use by non-smokers and by young people.
Consumers do not want medical regulation. There has been extensive
comment on the proposals on social media sites, Twitter, and letters to members
of the European Parliament – current users insist that these are consumer
products, a safe way of enjoying nicotine, rather than a therapy. These products are
popular precisely because they are not medicines. As one user put it – these are
not ‘smoking cessation products’, they are ‘smoking sensation products’.
Getting the balance right
The problem is the trade-off between making the product safe enough, but also
sufficiently attractive to achieve widespread uptake. On balance more weight
should be given to attractiveness, given their relatively low risks and the huge
consequences of continued smoking.
The argument that these products are currently unregulated is false. The
Electronic Cigarette Industry Trade Association has shown that they are covered
by the General Product Safety Directive and various other EC directives covering
electrical safety, chemical safety, weights and measures, packaging and labelling,
commercial selling practice and data protection.
Applications to MHRA are costly, including the licence fee and the required
studies, analyses, documentation. There are fears that few companies will be able
to afford this, that
the process will
favour big players and
drive many products
off the market. There
are further problems
with medical
regulation in that e-
cigarettes include a
big range of products
and product
combinations. Not all
of them are simple
pre-packaged
cigarette lookalikes,
but many are customisable, where the user can vary the delivery device and the
nicotine strength and flavourings. It is likely that medical regulation will
prematurely limit the range of products and stifle innovation.
Even the announcement of future medical regulation has created uncertainty
among retailers, current and potential e-cigarettes users.
How this will play out is uncertain. Given that MHRA has put the deadline as
2016, by then there will be a much larger market and it will be harder to limit and
control products. Already there have been four successful legal challenges against
classing these products as medical products (two in Germany, and one each in
Estonia and the Netherlands).
At the end of the day the potential public health gains from e-cigarettes will
be determined by the decisions about how to regulate these devices. There is
right thinking about tobacco harm reduction, but a risk of making significant
mistakes in the way this is played out in regulatory frameworks.
The danger is a classic regulatory trap: making safer products harder to obtain
than their unsafe counterparts. The regulatory proposals are tougher on e-
cigarettes than on tobacco cigarettes. The framing of electronic cigarettes within
a regulatory context misses the point that the public health drive must be to
promote, endorse and facilitate their use.
DDN
Prof Gerry Stimson is visiting professor, London School of Hygiene and Tropical
Medicine and director of Knowledge-Action-Change.
OR E-CIGARETTES

